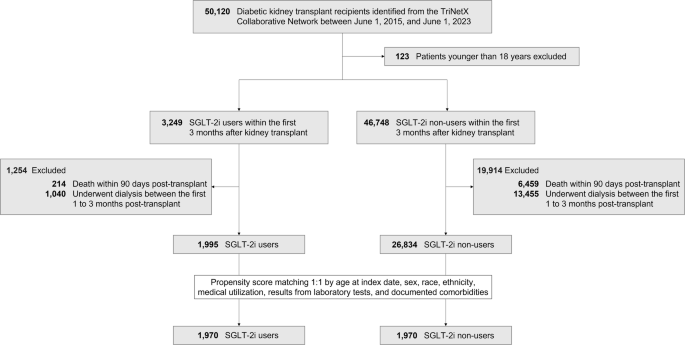
In current practice, it has been observed that only 6.5% of diabetic KTRs utilize SGLT-2i post-transplant. The all-cause mortality rate for SGLT-2i users showed a remarkable reduction of 6.56 per 1,000 person-years, suggesting that more frequent or routine use of SGLT-2i may improve survival in diabetic KTRs. This highlights the great potential of this. The reduction in MACE and MAKE incidence observed among SGLT-2i users is particularly noteworthy. The positive effects of SGLT-2i extend across different stages of baseline renal function, including patients with or without ACEi/ARB use, older or younger adults, obesity or not, and patients with different HbA1c levels. It has been suggested that these inhibitors not only have kidney-protective effects but also contribute to improving overall kidney health in diabetic KTR. This finding is of great importance given the increased risk of kidney-related complications in this population. Furthermore, our analysis revealed that the beneficial effects of SGLT-2i were consistent across different doses. This effect allows a wide range of patients, including those who require lower doses due to renal impairment or side effects, to still obtain significant health benefits. Specificity analysis further validated these benefits. The reduced frequency of dialysis cases in SGLT-2i users highlights the potential of these agents in preventing progression to severe renal complications in diabetic KTR.
Exposure control analysis emphasizes the reliability of our findings. The reduction in mortality and adverse events in patients treated with ACEi and ARBs, in contrast to the less pronounced variation in the vitamin C-exposed group, lends further confidence to our results. Additionally, our application: E-value indicates that a more substantial unmeasured confounder is unlikely to be needed to refute the estimated effect of the covariate, thus strengthening the argument about its causal impact. I am.17. Landmark analyzes involving starting follow-up periods at different time frames also showed a sustained benefit of SGLT-2i, even with intermittent use. This ensures the robustness of the results and reduces the possibility of guaranteed time bias or immortal time bias.18,19,20. Taken together, the above findings support the inclusion of SGLT-2i in the treatment regimen of diabetic KTR, considering that SGLT-2i can significantly reduce mortality and cardiac and renal adverse events. Masu. The importance of precision medicine is particularly emphasized in the management of patients with various health conditions and comorbidities.
Our study includes a large number of patients diagnosed with pretransplant diabetes and PTDM, which are major factors influencing morbidity and mortality. According to the 2024 international consensus on post-transplant diabetestwenty onePTDM increases the risk of graft loss, cardiovascular events, and death. Similarly, pretransplant diabetes predicts increased mortality and cardiovascular disease incidence. Kuo et al.4 found that pretransplant diabetes was a major predictor of all-cause and cardiovascular mortality, highlighting the importance of diabetes in transplant outcomes. Retrospective study of Tsai et al.twenty two Additionally, 427 KTRs demonstrated that pretransplant diabetic patients had a 6.36-fold higher risk of adverse long-term outcomes such as serum creatinine doubling, graft failure, and patient survival compared to non-diabetic patients. . These studies confirm that diabetic status has a significant impact on KTR and highlight the need for targeted management strategies.
Recent developments in the treatment of type 2 diabetes, particularly SGLT-2i, have shown significant cardiorenal benefits.5,6,78. The significantly lower incidence of MACE in the SGLT-2i group is consistent with a growing body of evidence supporting the cardiovascular benefits of SGLT-2i in diabetic patients.10. The cardiorenal benefits of SGLT-2i are primarily derived from multiple interrelated mechanisms, including natriuresis and subsequent blood pressure reduction, which significantly enhances cardiac and vascular function.twenty three,twenty four,twenty five. These effects also aid in renal protection by increasing tubulo-glomerular efficiency. SGLT-2i has been observed to reduce intraglomerular hypertension and hyperfiltration, reduce albuminuria, and increase erythropoietin production, thereby slowing the progression of nephropathy.twenty three,twenty four,twenty five. Our study supports these results and shows that SGLT-2i effectively reduced the incidence of MACE and MAKE in KTR. SGLT-2i may emerge as an essential drug for long-term cardiorenal protection in diabetic KTR due to its wide range of benefits. Conversely, a previous meta-analysis by Wu et al. for SGLT-2i in type 2 diabetes found a cardiovascular protective effect in high-risk type 2 diabetes patients. However, these inhibitors were not observed to have a significant effect on the incidence of nonfatal myocardial infarction or unstable angina, and an increased risk of nonfatal stroke was noted.26. A recent meta-analysis of 42,568 participants also suggested that SGLT-2i reduces the overall incidence of MACE but does not significantly change the incidence of individual myocardial infarction or stroke events. has been.27. These findings are consistent with our study results and further support the importance of SGLT-2i in long-term cardiorenal protection.
In our study, SGLT-2i users showed higher initial HbA1c levels than non-users early post-transplant, but this difference persisted over time at 6-9 and 9-12 months. decreased, indicating successful long-term glycemic control. Long-term users particularly benefited from reductions in all-cause mortality and MACE compared to new users, highlighting pleiotropic effects.28 While the original treatment goal of glycemic control has been exceeded, new users continue to benefit from a reduction in MAKE, an important event after kidney transplantation.
Studies have also shown that SGLT-2i plays an important role in slowing the progression of diabetic kidney disease, as evidenced in multiple studies.7,8,9. These inhibitors have also shown efficacy in the management of acute kidney disease (AKD). In a study utilizing the TriNetX database, Pan et al. reported that AKD patients using SGLT-2i had significantly reduced mortality, MACE, and MAKE compared to non-users.10. This highlights the importance of SGLT-2i in reducing the risk of cardiovascular and kidney disease in patients with type 2 diabetes and AKD, and as our findings suggest, the benefits of KTR. may contribute to
However, experience in diabetic KTR is limited, primarily due to concerns about the risk of hypotension due to urinary tract infections and osmotic diuresis, leading to a lack of prospective trials in KTR.12, 29, 30. Nevertheless, recent studies have shown the efficacy and safety of SGLT-2i in diabetic KTR.31. Demir et al. In a study conducted at two institutions, no increased risk of genital or urinary tract infections was observed with post-transplant SGLT-2i use, while proteinuria was significantly reduced and there were no adverse effects on graft function. Not observed for more than a year.32. Similarly, our study also shows that the use of these agents does not increase the risk of urogenital infections or AKI, confirming their safety profile in this population. A multicenter collaborative study by Lim et al. KTR with diabetes showed improved outcomes related to all-cause mortality, death-censored graft failure, and serum creatinine doubling, with only a few experiencing a transient decrease in estimated glomerular filtration rate.11. A study by Sánchez Fructuoso et al. SGLT-2i treatment demonstrated significant improvements in body weight, blood pressure, blood sugar levels, and kidney function that were sustained over 6 to 12 months.12. These comprehensive findings suggest that SGLT-2i is not only effective in managing various health aspects of diabetic KTR, but also provides a valuable option for long-term care. SGLT-2i has become an important treatment option for diabetic KTR due to its role in improving cardiorenal outcomes and manageable safety profile.
The main strength of this study lies in its comprehensive investigation of the effects of SGLT-2i on KTR in diabetes. Utilizing extensive patient data from the TriNetX electronic medical record database, this study boasts a robust and diverse sample size, allowing for a thorough and nuanced analysis of the impact of SGLT-2i on this specific patient group. did. In addition, PSM and E– The value of our research methodology increases the reliability and validity of our findings and provides a stronger basis for our conclusions. However, it is important to recognize that certain limitations may affect the interpretation of the results. First, relying on diagnostic codes to identify conditions introduces the risk of ascertainment bias, which may result in the underestimation of certain conditions. Second, the retrospective nature of this study, dependent on diagnosis and treatment codes, is susceptible to misclassification bias and residual confounding, and despite the implementation of rigorous methodology and variable validation strategies, these cannot be completely excluded. Third, exclusion of cases with incomplete outcome data may induce selection bias. The high number and percentage of excluded cases is primarily due to our strict criteria to ensure data integrity. To avoid potential bias and inaccuracy in morbidity classification, we excluded all patients with dialysis-related diagnosis or procedure codes within 1 to 3 months after transplantation. This may be the primary cause of the high post-transplant mortality and morbidity observed in this study. Fourth, the lack of specific dates for SGLT-2i use within the first 90 days post-transplant limits the ability to fully assess the impact of early use. Furthermore, although biases such as selective prescribing for patients with recurrent UTIs have been taken into account, direct evidence regarding decision-making for SGLT-2i use remains unavailable. Fifth, persistent time bias represents a limitation of our study, as the discrepancy between the length of the follow-up period and the duration of SGLT-2i use could distort the results. To address this, we conducted landmark analyzes over different selection periods to ensure consistent and robust results. Finally, it is important to note that our data source is aggregate rather than individual-level data. This limits the adoption of more nuanced designs, such as randomized controlled trial emulation, matching of prescription time distributions, and time-varying exposure models (similar to clone sensor weight approaches).33. We are generally concerned about using aggregated data to conduct comparative effectiveness studies. However, we recognize that no other database provides this level of detail and that some evidence is better. To compensate for this limitation, we conducted extensive sensitivity analyzes including longitudinal risk assessments at multiple time points to ensure the robustness and reliability of our findings despite the constraints of using aggregated data. did.
In conclusion, our observations suggest that the use of SGLT-2i in diabetic KTR is associated with a lower risk of all-cause mortality, MACE, MAKE, and lower dialysis incidence, suggesting a useful renoprotective effect. It shows the role. These findings highlight the potential of SGLT-2i in enhancing post-transplant care and suggest a transformative impact on treatment strategies. However, although our results strongly suggest a benefit for these agents, it is essential to conduct formal randomized controlled trials to conclusively confirm these findings. Future prospective studies should further investigate the long-term efficacy and safety profile of SGLT-2i in diabetic KTR, thereby expanding our understanding of the effects of SGLT-2i on different at-risk populations. Considering the great potential of SGLT-2i, we advocate the judicious clinical use of SGLT-2i in combination with a personalized approach to optimize the outcome of diabetic KTR.