The antibody treatment emapalumab helps children fare better after stem cell transplants to treat the rare blood disease hemophagocytic lymphohistiocytosis (HLH), according to clinical study results reported in the same journal. blood.
the studypublished online on August 27, 2024, led by first author Dr. Bethany VerkampDepartment of Hematology-Oncology, Cincinnati Children's Hospital, corresponding author Dr. Michael B. JordanDepartment of Immunobiology. Co-authors include Pierce Keyser; Sonata Jodere, MD, Dr. Anthony Sabulskyand Dr. Rebecca Marsh.
What is HLH?
HLH is caused by the uncontrolled activation of lymphocytes and macrophages, which flood the bloodstream with large amounts of inflammatory cytokines in a so-called cytokine storm. If HLH is not recognized and treated promptly, it can lead to multiple organ failure and death.
Primary or familial HLH occurs approximately 1.2 cases per million children per year. Approximately 20% of these children die within the first 2 months after diagnosis. Secondary or acquired HLH can occur at any age and is thought to result from temporary immune system disturbances such as severe infections. Mortality after diagnosis of secondary HLH is considered low but remains significant.
Cincinnati Children's Hospital is the most experienced facility in the country in treating HLH. Experts here have been working for years to improve outcomes for these children.
Treatment currently includes chemotherapy, corticosteroids, and the use of emapalumab to manage an overactive immune system.
Most patients with familial HLH require stem cell transplantation for long-term cure. Reduced-intensity conditioned hematopoietic stem cell transplantation (RIC-HSCT), here developed by the Bone Marrow Transplant Program, uses lower doses of chemotherapy compared to standard transplantation, resulting in lower survival rates during transplantation. It will be expensive. However, this improvement comes at a price. In a process called mixed chimerism and graft loss, the patient's own bone marrow may regrow and crowd out the donor's stem cells. Both conditions are dangerous and may require dangerous interventions to save the graft or restart a second stem cell transplant.
What is emapalumab?
Emapalumab was approved in 2018 as the first Food and Drug Administration (FDA)-approved treatment for primary HLH. Jordan was the U.S. principal investigator on the international clinical study that led to FDA approval.
Unlike chemotherapy, emapalumab blocks the inflammatory effects of interferon gamma (IFN-γ) but does not suppress bone marrow. For many patients, this can be the difference between surviving until transplant or not.
New research further documents the value of antibody treatments in supporting improved outcomes of stem cell transplants.
Improving intervention-free survival rates
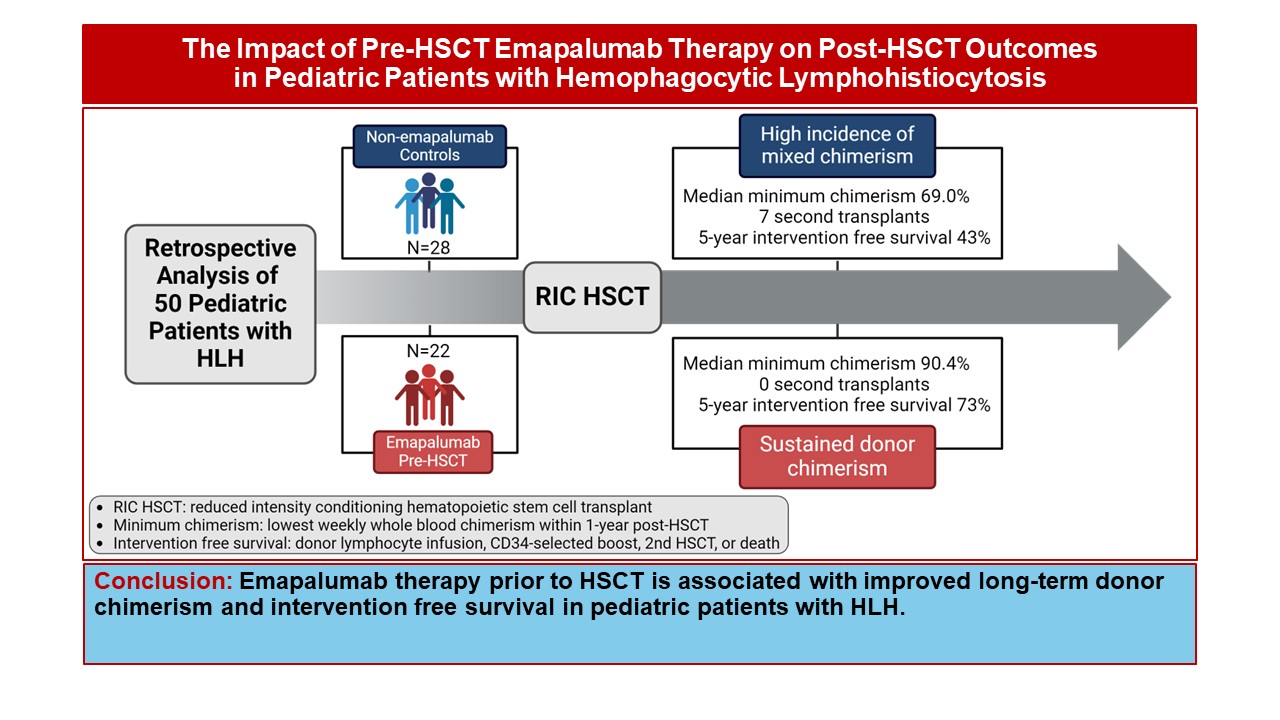
The clinical study involved 50 HLH patients who underwent stem cell transplantation between 2014 and 2022. This study compared the outcomes of 22 patients who received emapalumab before transplant conditioning therapy and 28 patients who did not.
Use of emapalumab was associated with a significant reduction in the incidence of mixed chimerism (48% vs. 77%) and severe mixed chimerism (5% vs. 38%). Most importantly, more children experienced intervention-free survival with antibody treatment (73% vs. 43%).
The improvement was even more pronounced in infants, the group most at risk for mixed chimerism. Of these patients, 75% achieved intervention-free survival with antibody treatment, compared with 20% without treatment.
“Emapalumab provides a solution to a particularly difficult clinical problem, and we hope these discoveries will change the practice of clinicians treating children with HLH,” Jordan says.
Looking forward, further research is needed to better understand how emapalumab changes stem cell transplantation and whether it may have a beneficial impact on patients undergoing HSCT for other diseases. is required.

