August 17, 2021
Weight loss is associated with improvement in patients with nonalcoholic steatohepatitis (NASH) and other favorable outcomes in the general population and may increase eligibility for liver transplantation in patients with cirrhosis and morbid obesity. However, finding safe and effective weight loss strategies for these patients can be difficult.
In a review paper published in Hepatology in 2021, Kimberly D. Watt, MD, and her coauthors study four Food and Drug Administration (FDA)-approved weight loss drugs and three in patients with and without cirrhosis. Discusses the use of different types of alternative medical treatments. Patients who have undergone liver transplantation. Dr. Watt is a hepatologist at the Mayo Clinic in Rochester, Minnesota, and the William J. Fong Liebig Center for Transplantation and Clinical Regeneration, where he serves as medical director of liver transplantation.
background
nash It is the most common indication for liver transplantation in women and the second most common indication in men.The relationship between obesity and the development of obesity nash Well established. In a recent analysis of national registry data, researchers noted that 35.9% of adult liver transplant recipients were obese at the time of transplant. And three years after liver transplantation, the obesity rate in these people increased to 40.8%.
Medications for weight loss, including dietary adjustments, exercise, and medications, are usually recommended for patients with a BMI (Body Mass Index) of 30 to 40. Surgical and endoscopic bariatric procedures are typically considered for patients with the following symptoms: BMI Patients over 40 years of age or with metabolic comorbidities and BMI However, in some patients, BMI Those that are within the range proposed for obesity treatment have additional risk factors and may not be suitable candidates for this approach. Medical approaches to reduce weight may be preferred in cirrhotic patients and liver transplant recipients who are at high risk of perioperative decompensation and mortality.
FDA-approved drug treatments for obesity
The four currently approved weight loss drugs are: F.D.A. Treatments for obesity include orlistat, phentermine-topiramate, naltrexone-bupropion, and liraglutide. Although some academic guidelines approve the use of these drugs in patients with cirrhosis or liver transplant recipients, they are not yet widely used in this population.
Dr. Watt and his co-authors point out that there is a lack of data regarding the use of these drugs in patients with cirrhosis and in the post-transplant setting. In their paper, the authors review the mechanisms of action and available safety and efficacy data for orlistat, phentermine-topiramate, naltrexone-bupropion, liraglutide, and three alternative therapies (cardamom, curcumin, and carnitine). , and is applying this to liver cirrhosis and transplantation. population. Their overarching conclusion is that F.D.A.-Approved drugs can be used in patients with well-compensated liver disease and transplant recipients, but may not be appropriate for patients with decompensated liver disease. Alternative agents were not thought to produce significant weight loss effects and their use was limited.
Dr. Watt and his coauthors describe the beneficial effects of weight loss drugs and alternative drugs on body weight and other metabolic substitutes, and outline suggested therapeutic pathways for weight loss pharmacotherapy in patients with obesity and cirrhosis. Masu. The care pathway begins with an assessment that identifies the patient’s Child-Pugh Score (CPS), transplant need and eligibility, current medications, and comorbidities.Class A and Class B patients have separate care pathways CPS For class C patients CPS. Additionally, the authors focus on the first-line use of glucagon-like peptide-1 (GLP-1) agonists due to their additional benefits in glucose management and provide an algorithmic approach to these agents in transplant populations.
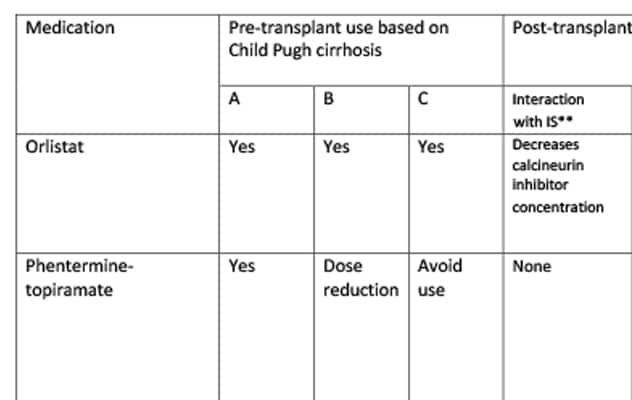
Guidance on the use of weight loss drugs
This review also summarizes guidance on the use of debulking or alternative drugs in patients with cirrhosis and liver transplant recipients. For each drug discussed, pre-transplant use (based on Child-Pugh classification), post-transplant use, dose adjustments based on renal status and common adverse events are listed in the table.
summary
all four F.D.A.-Approved weight loss drugs appear to have a variety of metabolic benefits that may impact both obesity and metabolic disease in certain patients. According to Dr. Watt and his co-authors, the choice of weight loss drugs for patients with cirrhosis and liver transplant recipients should be tailored to each patient’s clinical profile and should be followed carefully.
Dr. Watt said the small pool of data from patients with advanced liver disease and liver transplant recipients means that additional research is needed to provide more definitive data on the clinical impact of these drugs in these populations. We acknowledge that this means research is needed. However, this review answers several questions regarding the safety and potential impact of weight loss pharmacotherapy in these populations, which may inform future research.
“We know that for patients in these populations who need to lose weight, the mainstay remains diet and exercise. When these drugs are used in conjunction with diet and exercise regimens, patients can achieve moderate weight loss. We hope that future research collaborations will help answer more questions about how best to help these patients achieve weight loss. ,” says Dr. Watt.
For more information
Brown SA, et al. Pharmacotherapy for weight loss in liver cirrhosis and liver transplantation: translating data and underutilized potential. Hepatology. 2021;73:2051.

